
Answer to Solved RT B 2. The compressiblity factor for a gas is

The compressibility factor of a gas is defined as Z=P V / R T. The compressibility factor of idea

The compressibility factor of a van der Waals gas the critical point is equal to
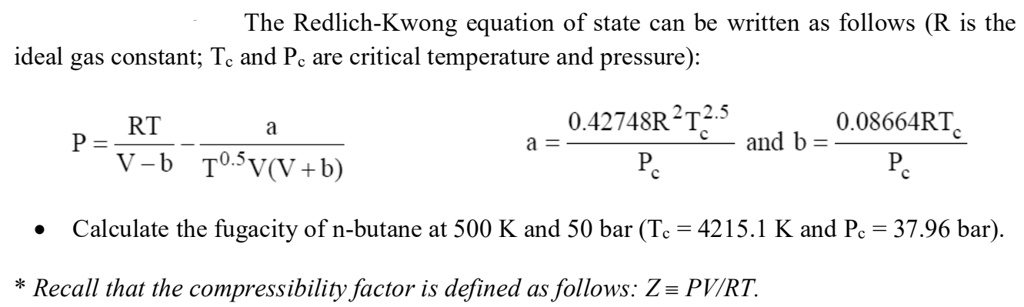
SOLVED: The Redlich-Kwong equation of state can be written as follows (R is the ideal gas constant; Tc and Pc are critical temperature and pressure): RT/P = V - b/(TOSV + b)

Gas Compressibility - an overview

Non-ideal behavior of gases (article)
A scientist proposed the following equation of state $p= ra

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Compressibility factor for methane.

OneClass: 2. Fugacity for a van-der-Waals gas Let's get a feel for how much fugacity deviates from pr

A certain gas obeys P(Vₘ - b) = RT. The value of (∂Z/∂P)ₜ is xb

The compressibility factor for a real gas at high pressure is :- (1) 1-pb/RT (2) 1+RT/pb (3) 1

Compressibility factor (gases) - Citizendium







