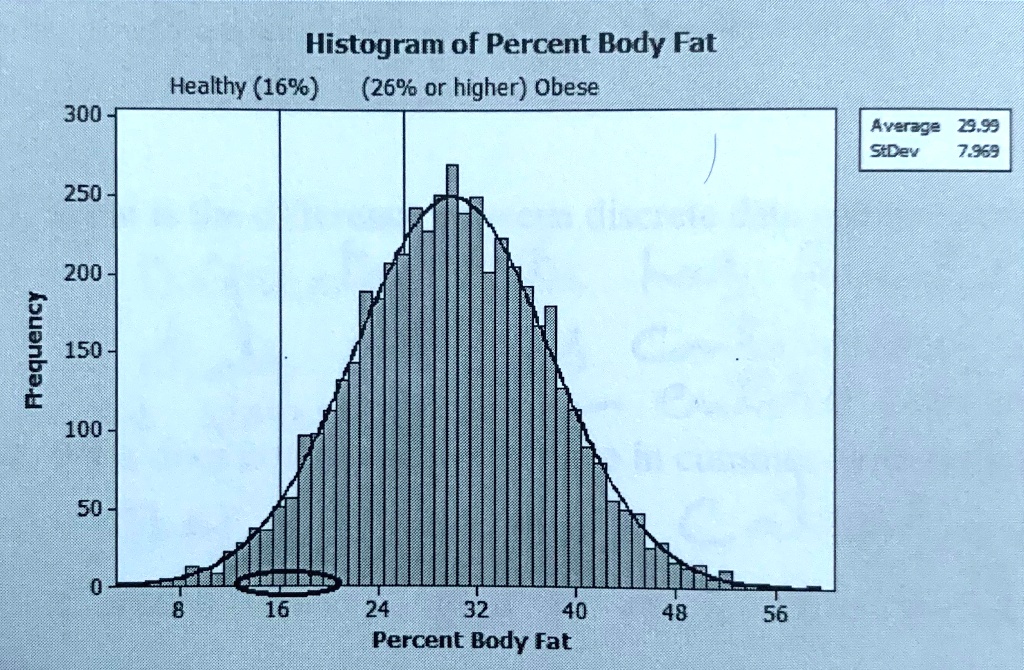
Solution For 13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g)
13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g) ₹ pressure. Then the ratio of the final volume to the initial volume of gases is:
Video solution 1: 13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g) ₹ pressure. Then the ratio of the final volume to the initial volume of gases is

Assobens Magazine #383 by ASSOBENS - Issuu

exh991-1q21investormeeti

Prova Fundação Hemocentro de BrasíliaDF - IADES - 2017 - para
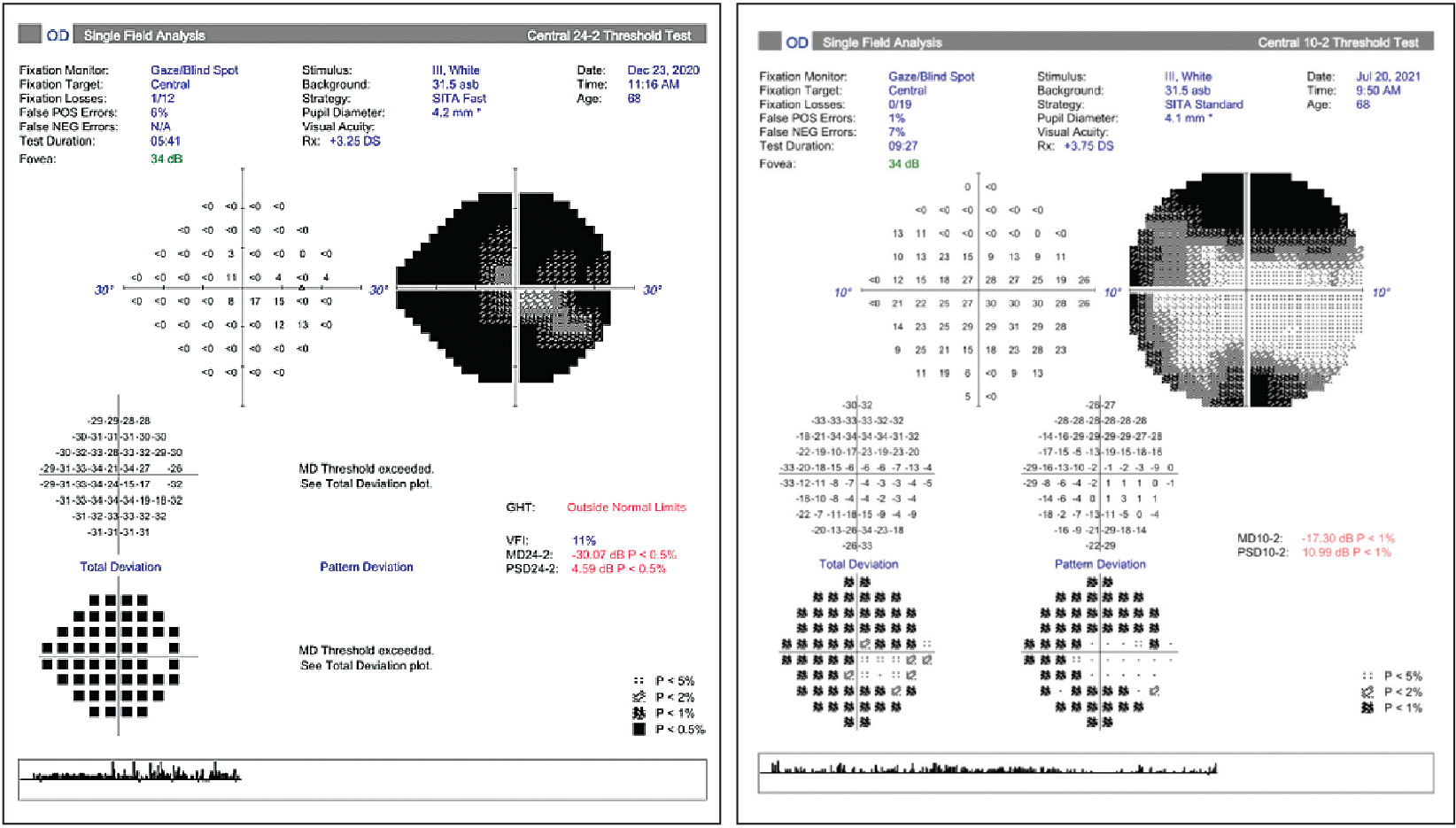
Prepping for the Glaucoma Grind
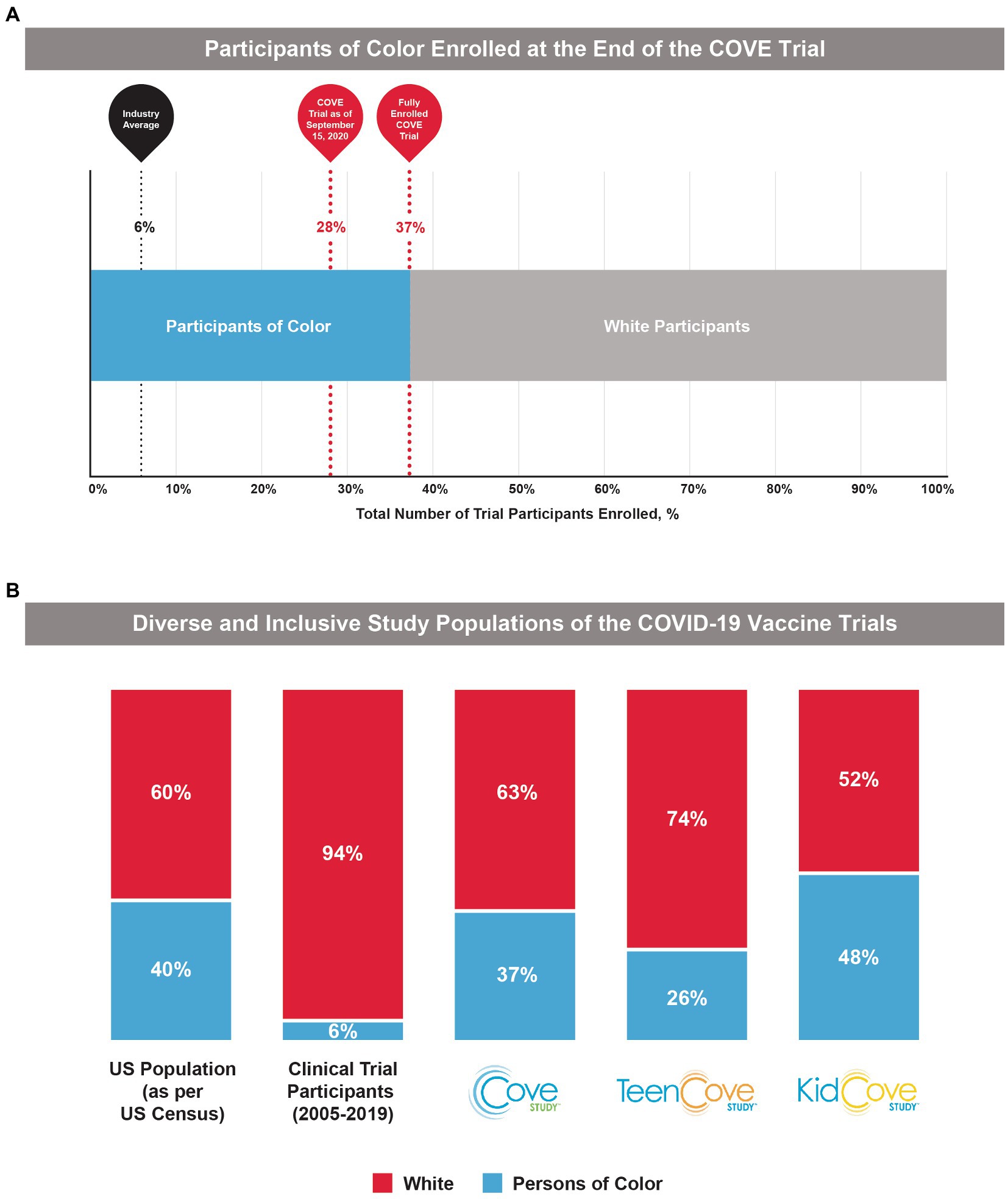
Frontiers Diversity and inclusion in clinical trials: Evolution
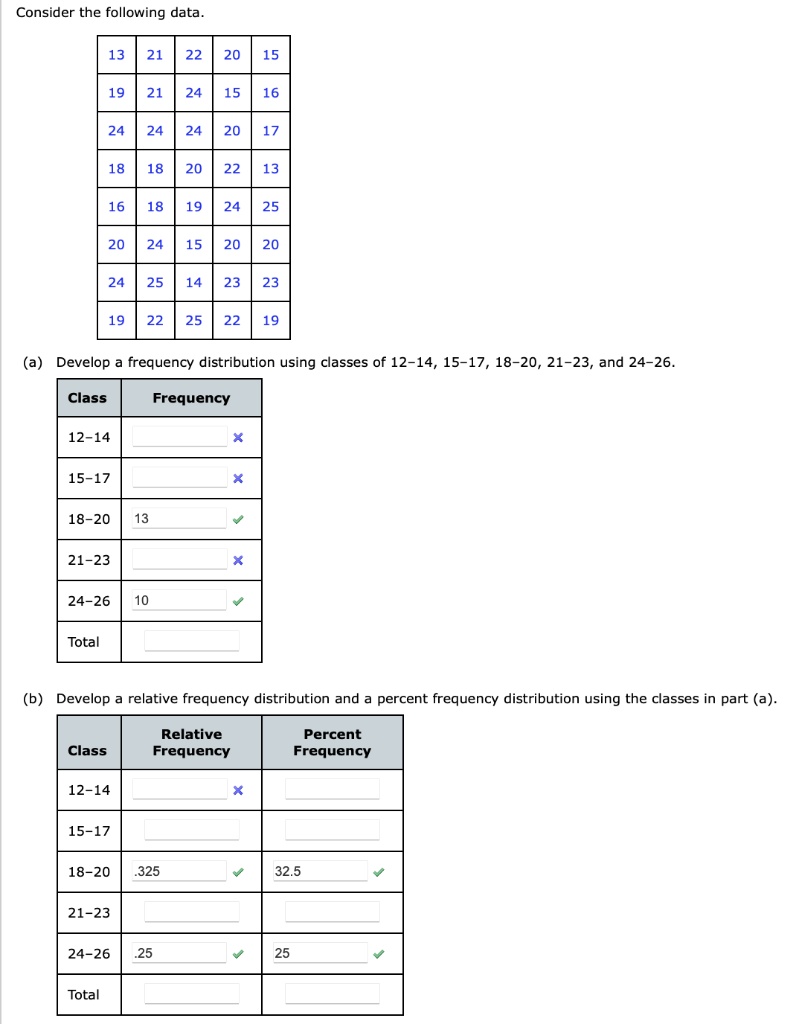
SOLVED: Consider the following data- 21 22 20 21 24 15 16 24 24 20

Update of the Brazilian Guideline for Familial
Solved 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27

Answer Key Transparencies
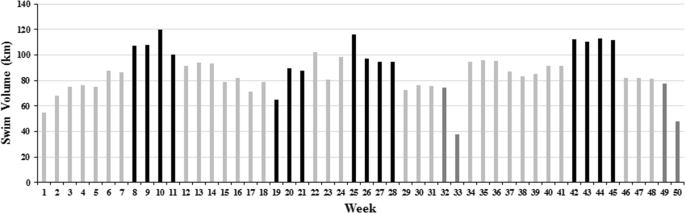
Contemporary Periodization of Altitude Training for Elite