
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

physical chemistry - Why does small value of van der Waals gas

physical chemistry - Why do some gases have lower value of Z for a

gas laws - Compressible Factor - Chemistry Stack Exchange
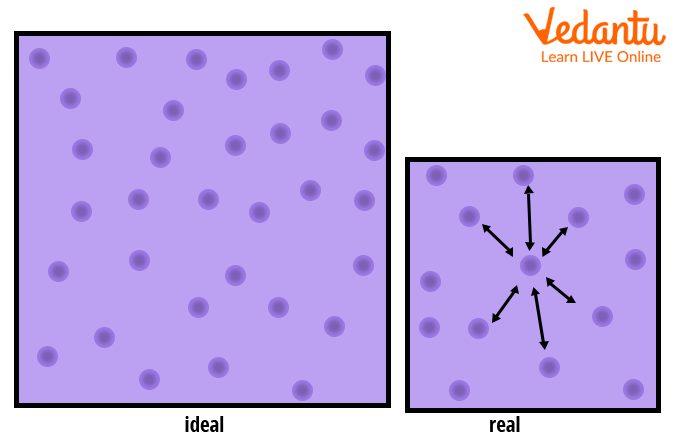
JEE - Compressibility Factor Important Concepts and Tips
Ideal gas - Wikipedia

gas laws - Which gas is easier to compress, the ideal gas or a

Strain engineering of two‐dimensional materials: Methods

Non-Ideal Gas Behavior Chemistry: Atoms First

3.2 Real gas and compressibility factor – Introduction to

Application of Machine Learning in Optimizing Proton Exchange

Ideal gas - Wikipedia







