
I found an increase of 3100J Have a look
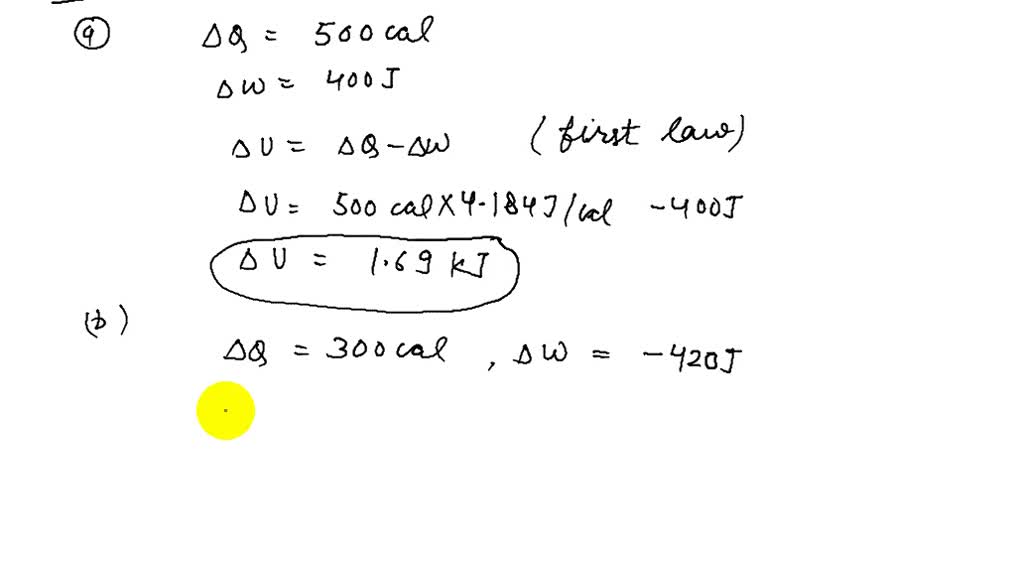
⏩SOLVED:In each of the following situations, find the change in

A system absorbs `600J` of heat and work equivalent to `300J` on

How to calculate ΔE when the system absorbs 250 J of heat energy

Charlotte Aaron Physics Tutor on HIX Tutor

Heat Transfer 10thEdition by JP Holman p

The elastic properties, elastic models and elastic perspectives of
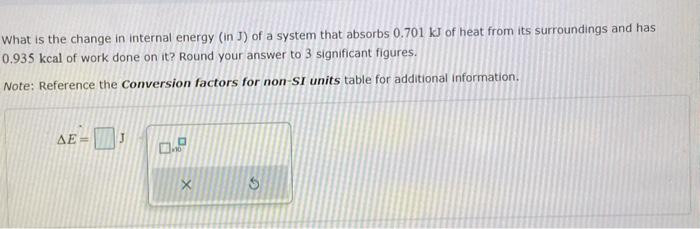
Solved What is the change in internal energy (in J) of a

SOLVED: attempts left Check my work Be sure to answer all parts
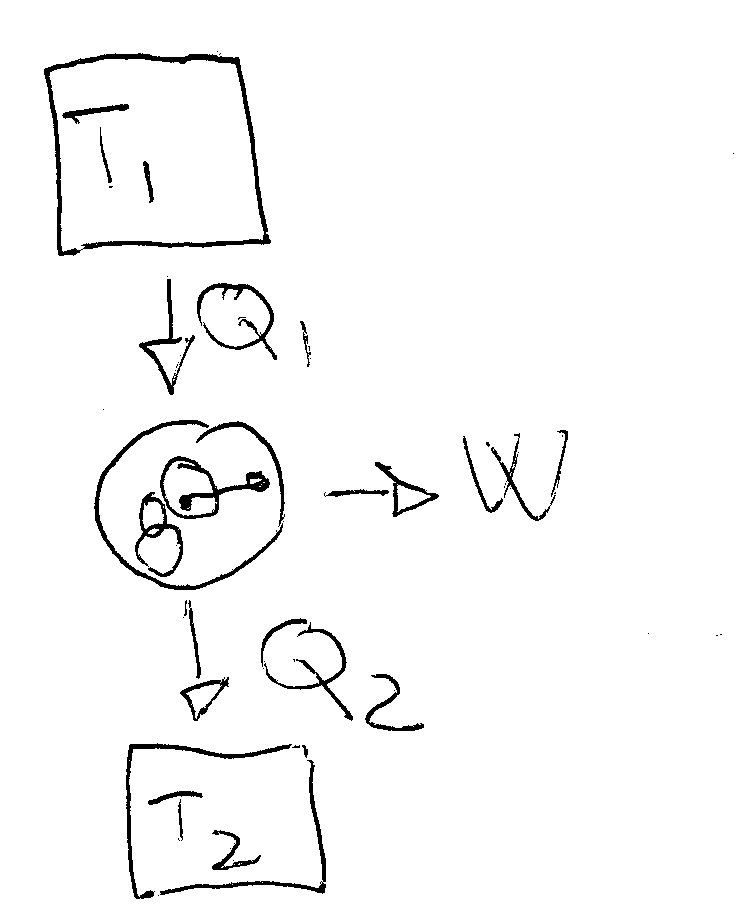
Heat and Work - Physics
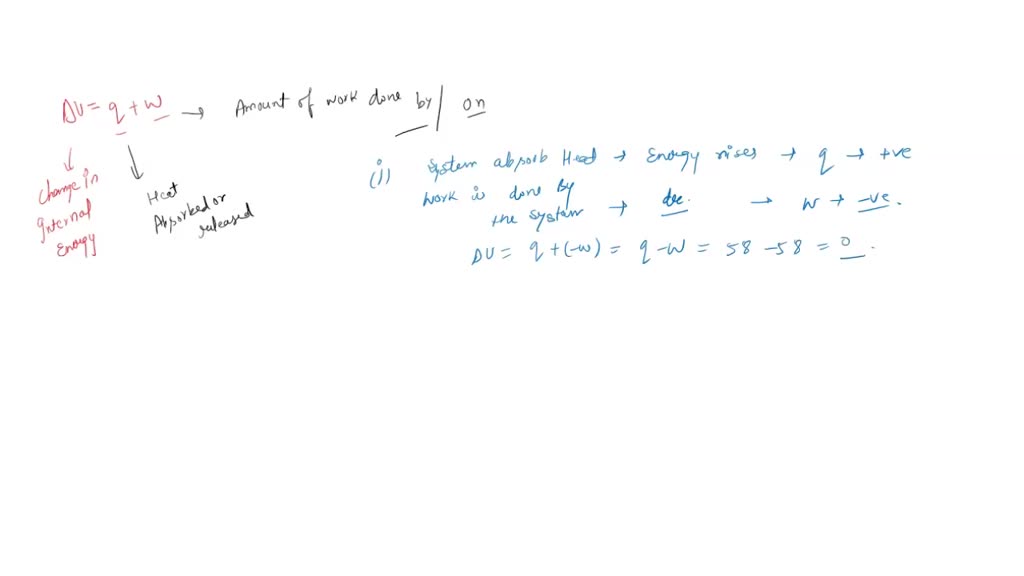
SOLVED: What is the change in internal energy of a system if the







