
Click here:point_up_2:to get an answer to your question :writing_hand:pick only the incorrect statement
Click here👆to get an answer to your question ✍️ Pick only the incorrect statement-for gas A- a-0-the compressibility factor is linearly dependent on pressure-for gas C-aneq 0-bneq 0-it can be used to calculate a and b by giving lowest P value-for gas B-0-if b-0-the compressibility factor is lineraly dependent on pressure-slope all three gases high pressure is positive
Solution- -C-xA0-for gas C-a-x2260-0-b-x2260-0- it can be used to calculate a and b by giving lowest P value-According to the real gas equation-The constants -apos-a-apos- and -apos-b-apos- are Van der Waals constant for attraction and volume for a given gas-The -apos-a-apos- values for a given gas are measure of intermolecular forces of attraction- More are the intermolecular forces of attraction- more will be the value of a-xA0-For a given gas van der Waals constant of attraction -apos-a-apos- is always greater than van der Waals constant of volume -apos-b-apos-xA0-The gas having higher value of -apos-a-apos-xA0- can be liquefied easily and therefore H2 and He are not liquefied easily-According to this- for gas A-Z-gt-1-a-0 and its dependence on P is linear at all pressure and for gas B-Z-lt-1-b-0 and its dependence on P is linear at all pressure-Also- at high pressure- the slope is positive for all real gases
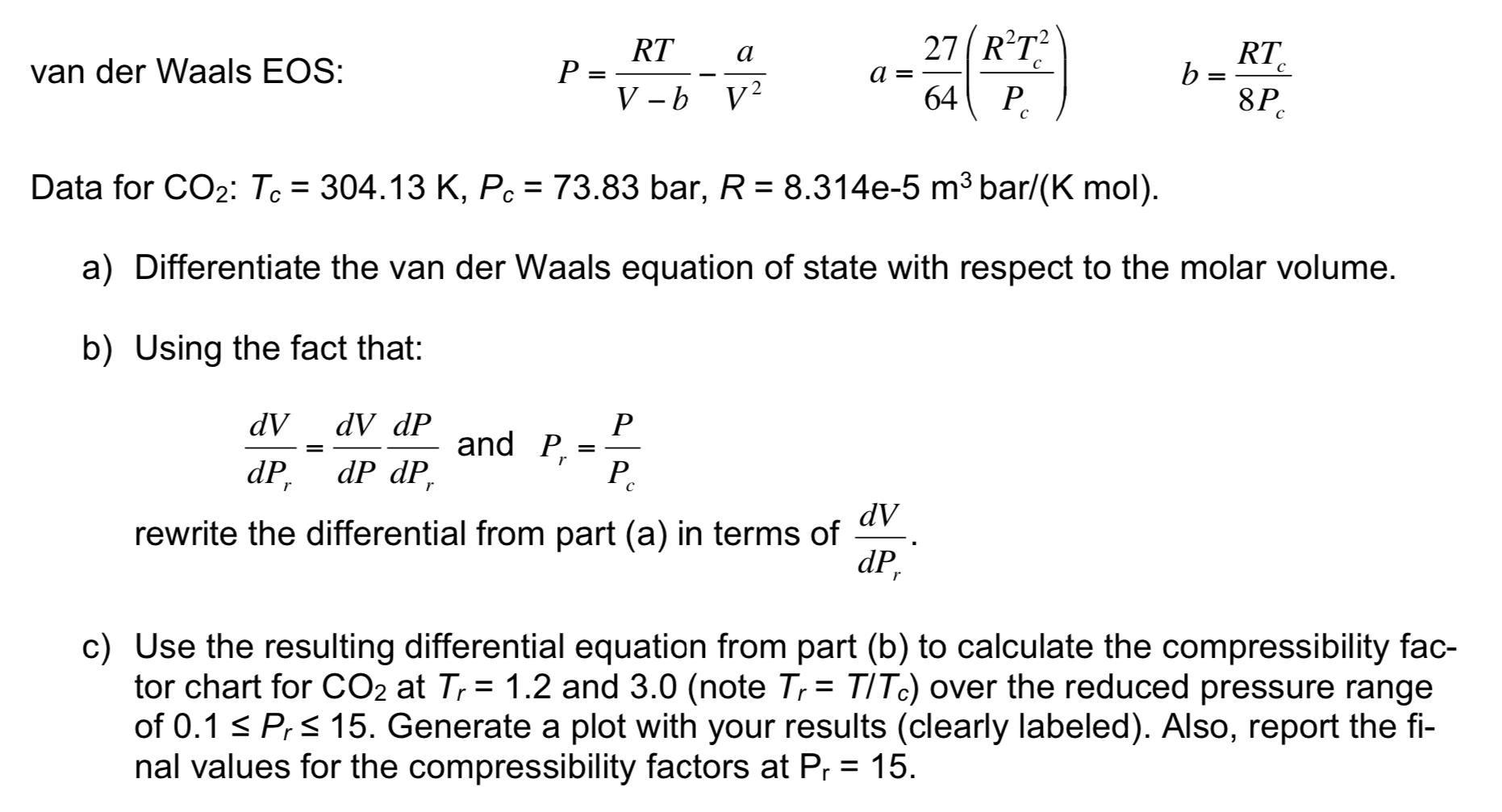
Solved The van der Waals equation of state can be used to

Which of the following statement is wrong ? For gas A, a= 0 and z will linearly depend on pressureFor gas B, b = 0 and z will linearly depend on pressureGas

The given graph represent the variations of Z (compressibility factor (Z)=dfrac {pV}{nRT}) versus P, three real gases A, B and C. Identify the only incorrect statement.For the gas B, b=0 and its
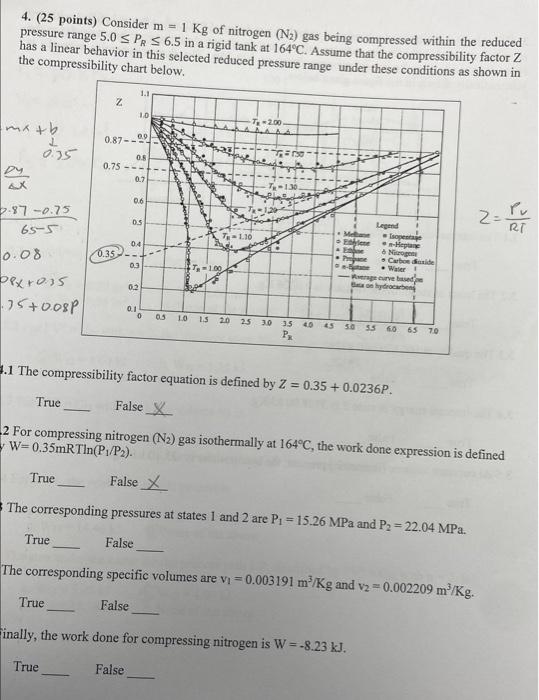
Solved 4. (25 points) Consider m=1Kg of nitrogen (N2) gas

Analysis of some factors affecting the water vapour diffusion in soils

Solved Please answer all the questions and explain how the

Liquid 116. Which of the following is the only incorrect statement - Ideal gas X L (1) For the gas (A): 0 and it varies linearly with P Hut2) For the gas (

Pick only the incorrect statement.for gas A, a=0,the compressibility factor is linearly dependent on pressure.for gas C,aneq 0,bneq 0,it can be used to calculate a and b by giving lowest P value.for
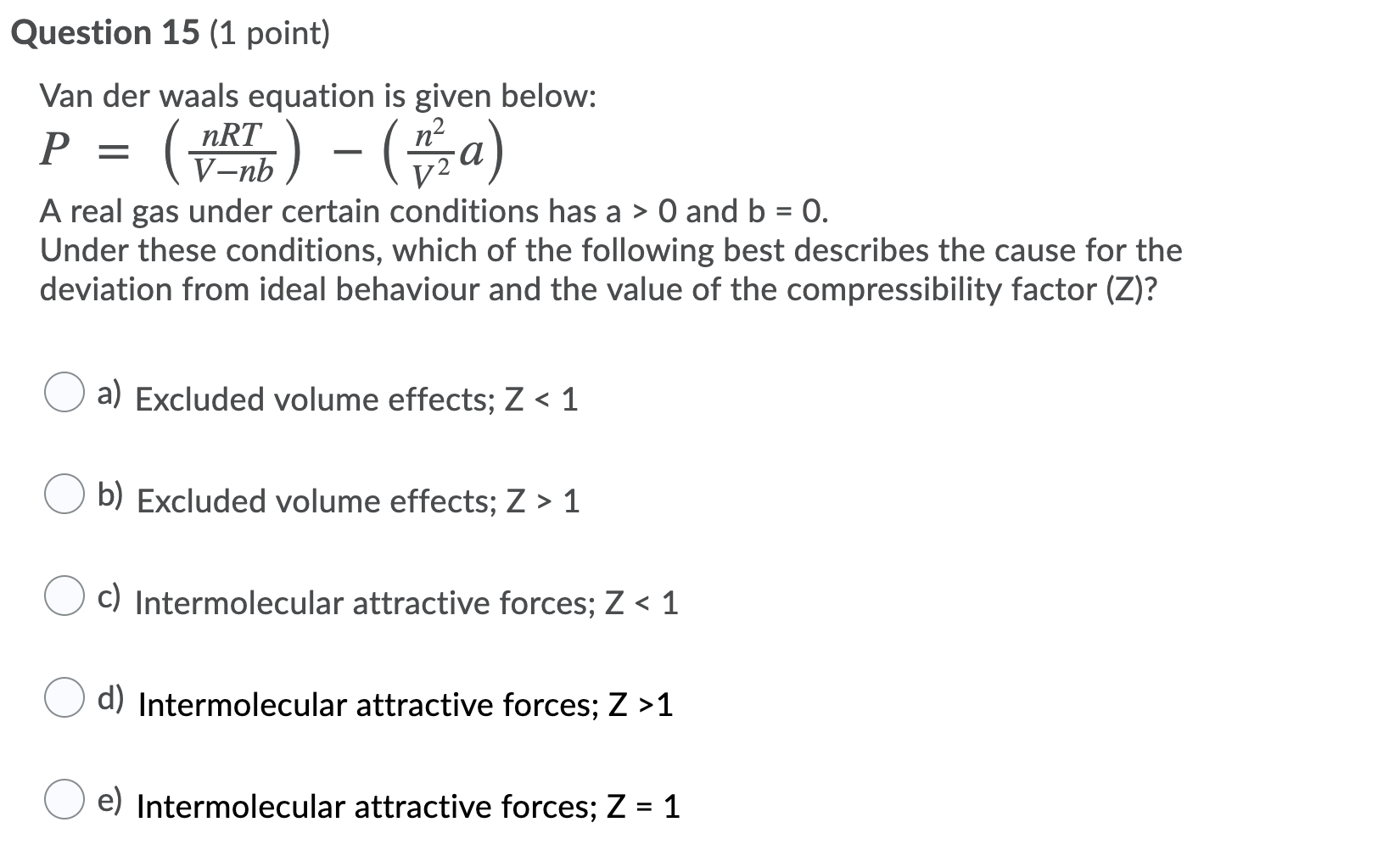
Solved Question 15 (1 point) Van der Waals equation is given

variations of 2 12.7 (a) eb (c)-(ar (d) - 6. The given graph represent the variations (compressibility factor (Z)=- gases A, B and C. Identify the only incorrect statement pl) versus p

The given graph represents the variation of Z (compressibility factor =) versus P, for three real gases A, B and C. Identify the only incorrect statement. [JEE 2006]a)For the gas A, a =





