
Overview Brass also has excellent thermal conductivity making it a first choice for heat exchangers (radiators). Brasses have a range of attractive colours ranging from red to yellow to gold to silver. With the addition of 1% manganese, brass will weather to a chocolate brown colour.
Brass does not become brittle at low temperatures like mild steel..
Brasses have a range of attractive colours ranging from red to yellow to gold to silver. With the addition of 1% manganese, brass will weather to a chocolate brown colour..
Predynastic Egyptians represented it by the ankh symbol, also used to denote eternal life. The Greeks knew brass as ‘oreichalcos’, a brilliant and white copper. Romans also liked it especially for the production of golden coloured helmets..
It conducts electricity so the electrons must be delocalized. There is no chemical formula for brass because it is an alloy and not a chemical compound. Brass is made by combining copper and zinc in an approximately 2 to 1 ratio. But there are a ton of different alloys, each with its own ratio of copper to zinc and each with its own mechanical properties..
Structure Examaple : YELLOW BRASS 60 % copper 40 % Zinc RED BRASS 85% copper 15% Zinc as a mineral however it would be Cu3Zn2.
The advantages of brass for architectural applications include its excellent corrosive resistance, and it’s joining, plating, polishing and finishing characteristics. Brass is easily machined. B r a s s i s c h e a p e r t h a n s t e e l !.
The biggest challenge to upkeep most metals, including brass, is the removal and inhibition of tarnish. All substances, especially metals, oxidize when exposed to the air..
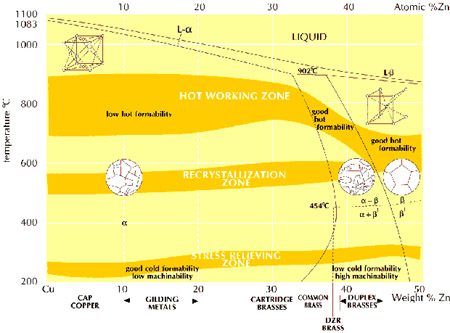
Applications EFFECT OF ALLOYING ADDITIONS Alloying additions are made to the basic copper-zinc alloys for a variety of reasons:- to improve machinability to improve strength and wear resistance to improve corrosion resistance for other special reasons The very wide variety of standard brass compositions that are available reflect the many ways in which an optimum combination of properties can be tailored to ensure fitness for the desired application.
For example, brass doorknobs disinfect themselves of many bacteria within eight hours. This effect is important in hospitals, and useful in many contexts..
High Corrosion Resistance : Nuts, Plumbing fittings, Gas fittings, Marine fittings. Free-Machining : Electrical components, Cable connectors, Jets, Injectors, Unions, Terminals Cold working : Automotive components, Pins, Precision etching, Motion sensors..

Metals (non-ferrous)

Minerals, Free Full-Text

Electroplating 101: How Plating Metals Works
Innovations: Introduction to Brasses (Part II)

Custom Welding Fabrication Services, Maintenance, Repair

PPT – Properties and Uses of Metals PowerPoint presentation

Copper - Wikipedia

Electroplating 101: How Plating Metals Works
Using which property of metal are metal sheets formed? - Quora
4. Materials - Engineering for Industrial Designers and Inventors [Book]







