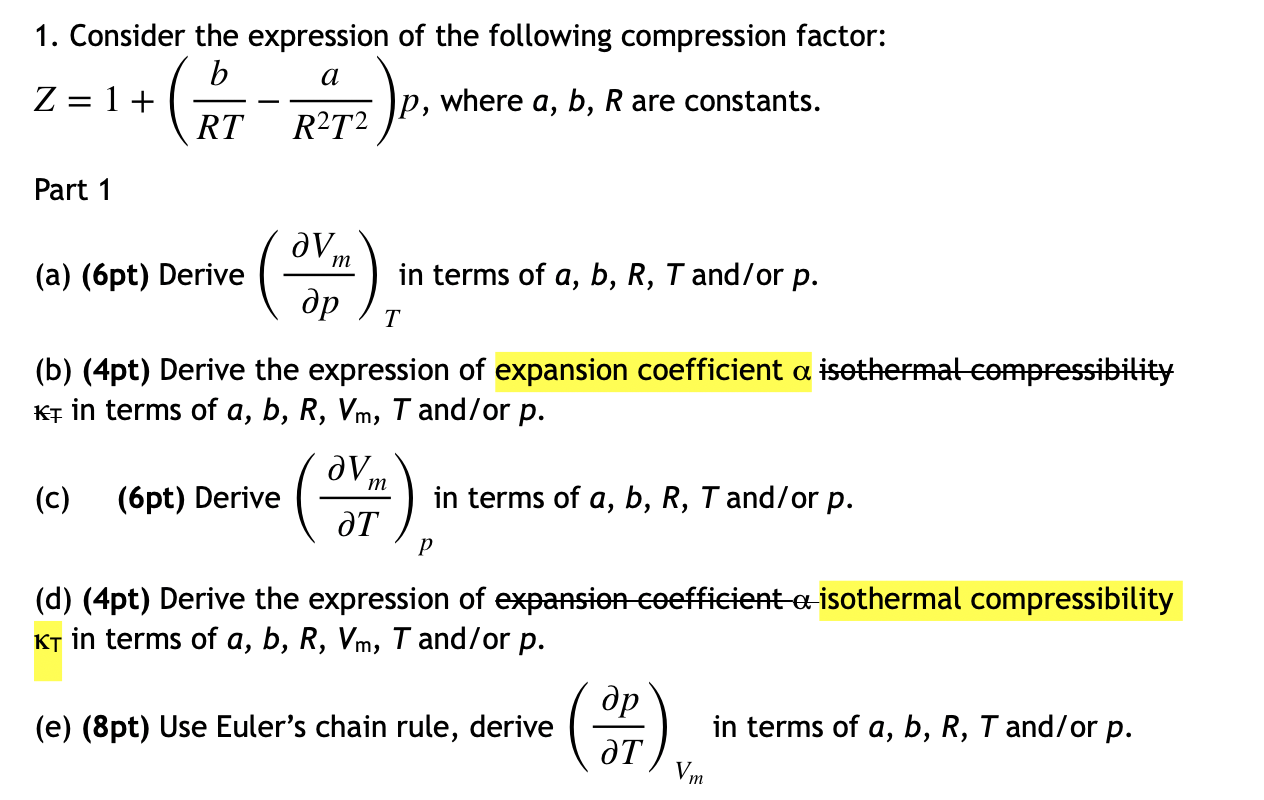
Click here:point_up_2:to get an answer to your question :writing_hand:unubat boyle temperature the value of compressifactor z has a value of one over a
Click here👆to get an answer to your question ✍️ UNUB At Boyle temperature- the value of compressi factor Z has a value of one over a wide range of pressure- This is due to the fact that in the van der Waals equation -1- The constant a is negligible and not b -2- The constant b is negligible and not a -3- Both the constant a and b are negligible -4- Attraction balances repulsion

qph.cf2.quoracdn.net/main-thumb-56835184-200-zlplu

C11 HSC Chemistry Text Book PDF, PDF

SOLVED: The compression factor Z reveals information about intermolecular interactions in real gas. Briefly describe how the values of compression factor Z, varies with pressure (i.e. at low moderate and high pressure).
The value of compression factor at the critical state of a vander
Solved I have a question about Boyle Temperature. I

The value of compressibility factor at the critical state the gas matches with the `Z_(c )` is
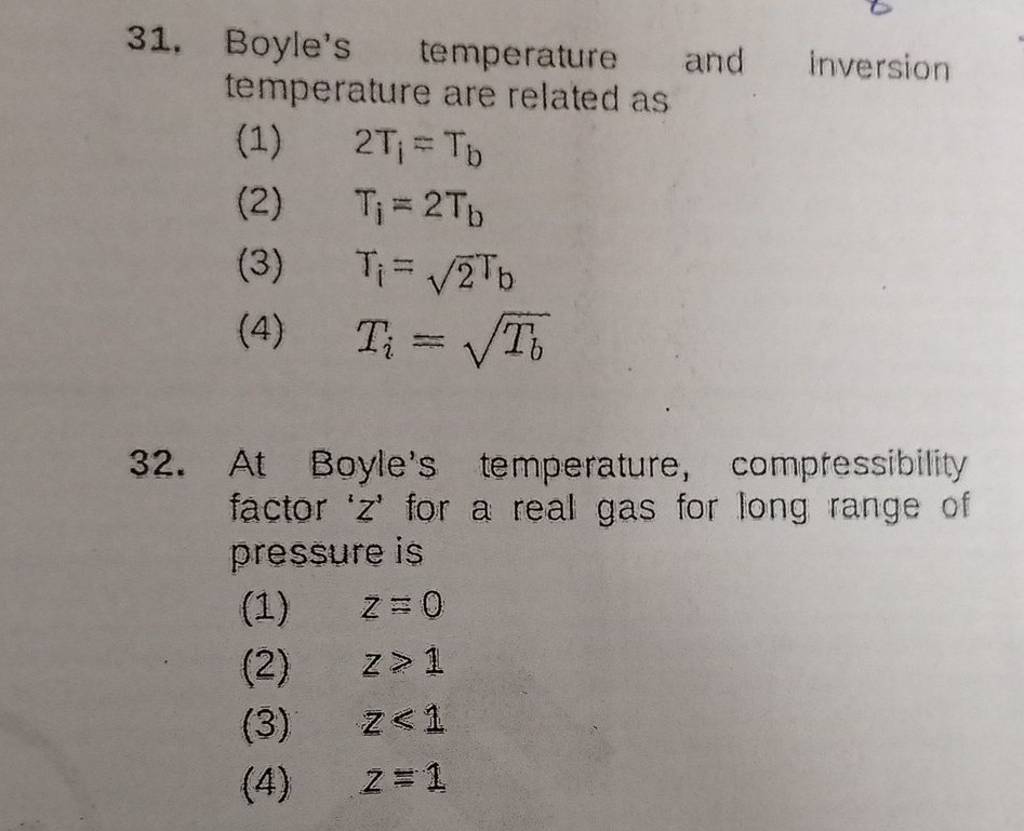
At Boyle's temperature, compressibility factor ' z ' for a real gas for l..

Solved The compression factor, Z, can also be calculated

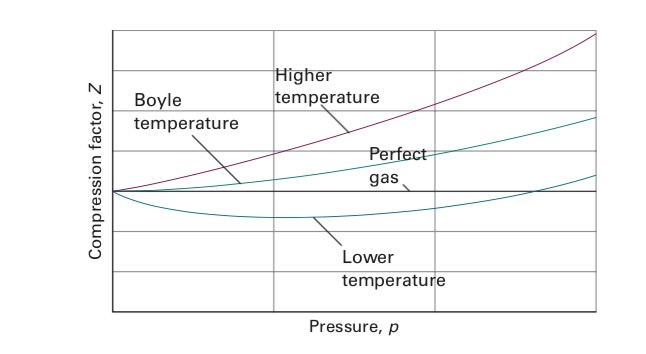
At Boyle's temperature, the value of compressibility factor Z = PV m / RT = V real / V ideal has a value of 1 , over a wide range of pressure.